Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the major curative immunotherapy for hematologic malignancies. The therapeutic benefits of allo-HSCT are primarily derived from an anti-leukemia effect or graft-versus-leukemia (GvL) that is mediated by mature T cells in donor grafts. Unfortunately, these T cells could react against recipient tissues resulting in graft-versus-host disease (GvHD), a life-threatening complication post allo-HSCT. Due to this strong association between GvL and GvHD, developing a more effective therapeutic strategy to selectively suppress GvHD while preserving or enhancing the GvL effects remains a fundamental yet unmet medical need.
We have previously published that co-blockade of interferon gamma receptor (IFNGR) and interleukin-6 receptor (IL6R) results in a complete prevention of GvHD (Choi et al., Leukemia, 2018). Moreover, in vivo administration of baricitinib, an inhibitor of Janus kinases 1 and 2 (JAK1/JAK2) which are downstream of IFNGR and IL6R, also prevents GvHD while preserving GvL in our preclinical murine model. The possible mechanistic pathways by which baricitinib mitigates GvHD include robust upregulation of regulatory T cells (Tregs), inhibition of GvHD-inducing Th1/Th2 cell differentiation, an altered T cell trafficking away from GvHD organs, and reduction of antigen presenting cells' allo-reactivity.
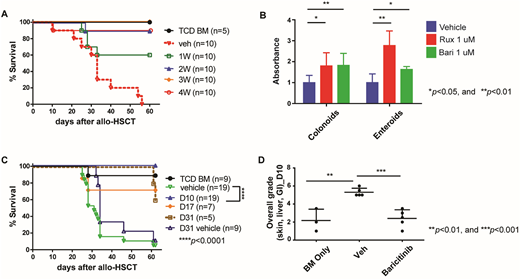
Given the potent modulatory effect of baricitinib on GvHD and GvL, we next sought to determine the minimum duration of baricitinib treatment for the complete prevention of GvHD. Since we have found that baricitinib significantly increases Tregs during the first two weeks of the treatment, we hypothesized that two weeks of baricitinib administration is sufficient to completely prevent GvHD. We performed allo-HSCT in which B6 (H-2b) T cell-depleted bone marrow cells (5x106) along with pan T cells (5x105) were intravenously injected into lethally irradiated Balb/c mice (H-2d). Baricitinib was subcutaneously administered to the recipient mice once a day (5 days/week) for a period of one, two, three, or four weeks following allo-HSCT. Overall survival rates, clinical GvHD scores, complete blood counts, immunophenotyping of blood cells and histopathological scores were assessed to determine whether the GvHD was prevented. We have found that two to four-week treatment groups demonstrate significantly better overall survival rates, blood count recovery and histopathological scores than vehicle control or the one-week treatment groups, while no such significant differences were observed among the other groups (Fig. A). All of these data suggest that two weeks of baricitinib treatment is sufficient for complete prevention of GvHD.
Lastly, we have previously reported that baricitinib reverses established GvHD (Choi et al., Leukemia, 2018) and we have recently found that both JAK1/JAK2 inhibitors, ruxolitinib and baricitinib, significantly enhance human intestinal organoid growth in vitro (Fig. B). Of note, the intestines are frequently diagnosed and severely damaged GvHD target organs in both human patients and our mouse models of GvHD. Thus, we hypothesized that baricitinib not only modulates immune cell functions as described above, but also promotes restoration of tissues damaged by GvHD. To test this hypothesis, we delayed baricitinib administration until mice developed clinically apparent GvHD and administered baricitinib starting on days 10, 17, or 31 after allo-HSCT for a period of two weeks. Despite this latency, the day 10 group showed 100% overall survival rate (5% in control, p<0.0001) while 70% and 60% in the days 17 and 31 groups, respectively (Fig. C). According to histopathological analysis of the three major GvHD target organs (GI, skin, liver) the day 10 group also demonstrated a significantly lower total grade than the vehicle control (Fig. D) whereas only a trend towards lower GvHD scores were observed in the rest of the groups. Hence, our data suggest that baricitinib reverses established GvHD by promoting tissue regeneration in GvHD damaged organs.
In conclusion, our studies demonstrate that baricitinib-induced blockade of IFNGR and IL6R represents a promising therapeutic strategy for both prevention and treatment of GvHD while preserving GvL. Molecular mechanisms underlying baricitinib-mediated reversal of established GvHD are being currently investigated and will be presented.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal